In the realm of physics, the pursuit of precision in capturing fleeting chemical events has long been a central challenge. Traditional methods brought forward significant advancements in our understanding, yet they fell short of illuminating the intricate processes that unfold on material surfaces in real time. Researchers at TU Wien (Vienna) have taken a substantial leap forward with their recent work on generating laser-synchronized ion pulses that last under 500 picoseconds, a development that promises to transform surface analysis and chemical observation.
As in photography, where a rapid exposure time is crucial for capturing fast-moving subjects, the same principle applies in the high-energy physics domain. The ability to observe rapid chemical reactions, especially at the atomic level, relies on reducing the temporal limitations of observation methods. Therefore, by harnessing the unique characteristics of ultrafast ion pulses, researchers can now delve deeper into chemical processes while they are actively occurring, rather than merely studying their aftermath.
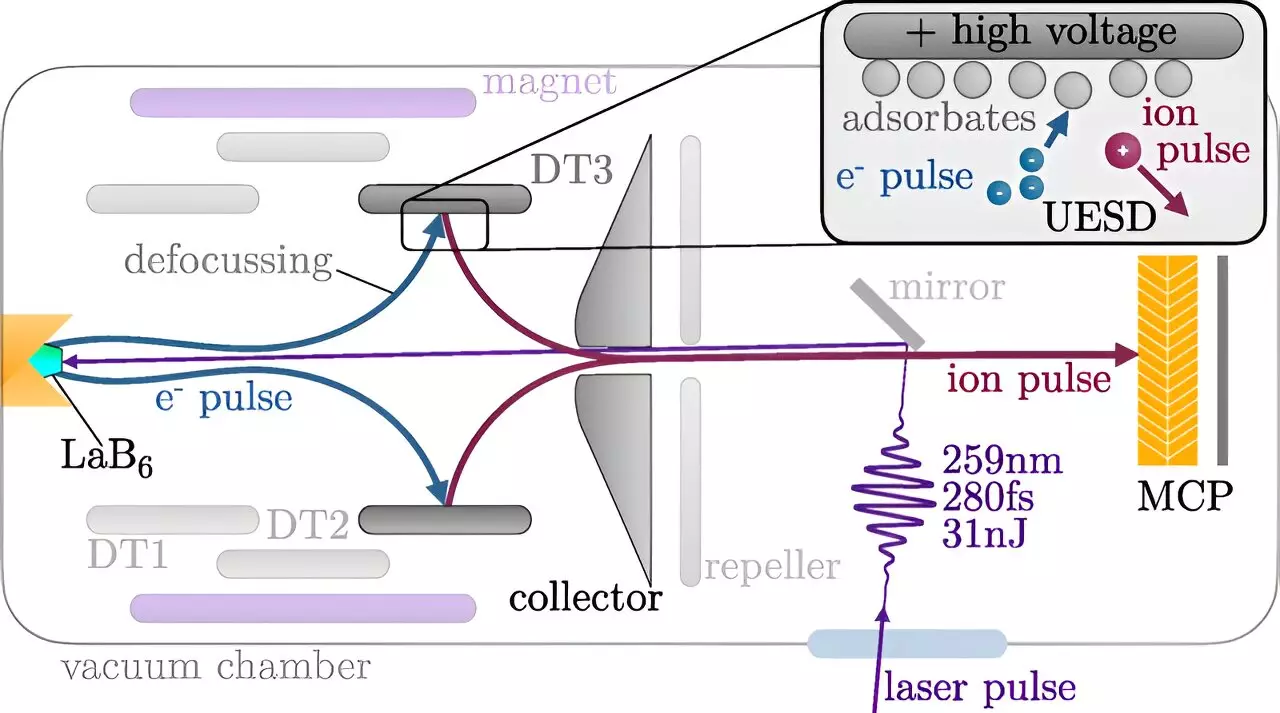
The fundamental breakthrough arose from a novel method of generating short and powerful pulses of ions. Historically, ion beams have been employed for various applications, including material analysis, surface cleaning, and modification, but the material transformation observed represented merely the end state of a more complex temporal evolution. Until recently, researchers struggled to produce ion pulses short enough to monitor the dynamic interactions taking place at the surface level.
The ion pulses created at TU Wien last under 500 picoseconds—a timespan that, while still longer than the quickest laser pulses, is significantly shorter than previously achievable durations. In practical terms, light can only travel a mere 15 centimeters in this timeframe, making it ideally suited for analyzing rapid changes on surfaces. The precision timeline achieved with this technology brings us into a new spectrum of surface analysis that was previously unattainable.
The technique for generating these ultrafast ion pulses is intricate yet elegant. It begins with the emission of electrons following the exposure of a cathode to a laser pulse. These accelerated electrons then bombard a stainless steel target, where they interact with a thin layer of atoms—specifically, hydrogen and oxygen—that are naturally present on the surface. This interaction results in a fraction of the atoms being ejected, allowing for a selection process that determines the ions’ subsequent trajectory.
This pivotal selection of ions serves to direct the resulting pulses with exquisite precision toward the target surface, enabling the researchers to time the impact of the ions relative to specific chemical reactions taking place—an unprecedented opportunity to probe chemical changes in motion. Prof. Richard Wilhelm of the Institute of Applied Physics at TU Wien highlights that this control over timing is a game-changer; it allows for the observation of phenomena at picosecond timescales, thereby providing insights into the sequential course of chemical reactions as they unfold.
While the initial exploration has focused on protons, the methodology has the capacity to extend to various types of ions, such as carbon or oxygen ions. This adaptability depends fundamentally on the choice of atoms that form the layer interacting with electrons, laying the groundwork for a rich array of analytical opportunities. Furthermore, the ability to generate electrically neutral atoms and negatively charged ions can enhance the investigative breadth of the research.
Looking forward, there is considerable potential to further decrease the ion pulse duration. By implementing alternating electromagnetic fields, researchers can optimize the speeds of ions within the pulse, allowing for an even shorter observation window. Wilhelm emphasizes that this technique not only provides an efficient means to understand ultrafast processes, but also establishes a new platform for integrating findings with existing ultrafast electron microscopy technologies.
The advancements made by TU Wien represent a pivotal moment in the field of surface chemistry and physics. The ability to generate and control laser-synchronized ion pulses on the order of 500 picoseconds heralds a new era in the real-time observation of chemical processes. As scientists continue to refine and expand this technology, we anticipate exciting developments that will deepen our understanding of chemical interactions at their most fundamental levels, potentially revolutionizing not only scientific inquiry but also practical applications across various sectors.