Transcranial focused ultrasound (TFUS) emerges as a groundbreaking non-invasive methodology aimed at enhancing neurological treatments. By utilizing high-frequency sound waves, TFUS offers the potential to target specific brain regions, thereby presenting promising solutions for various neurological disorders. One of the most significant implications of TFUS is in the treatment of drug-resistant epilepsy and recurrent tremor conditions, areas that have historically proven challenging for conventional therapeutic approaches.
Researchers from Sungkyunkwan University (SKKU), the Institute for Basic Science (IBS), and the Korea Institute of Science and Technology have made significant progress in brain sensor technology. Their recent work culminated in a new sensor designed for effective TFUS application in patients. This sensor, detailed in Nature Electronics, is engineered to adapt its shape dynamically, thereby achieving optimal adherence to the intricate contours of the brain’s surface. Such a feature allows for both the recording of vital neural signals and precise stimulation of targeted regions through low-intensity ultrasound waves.
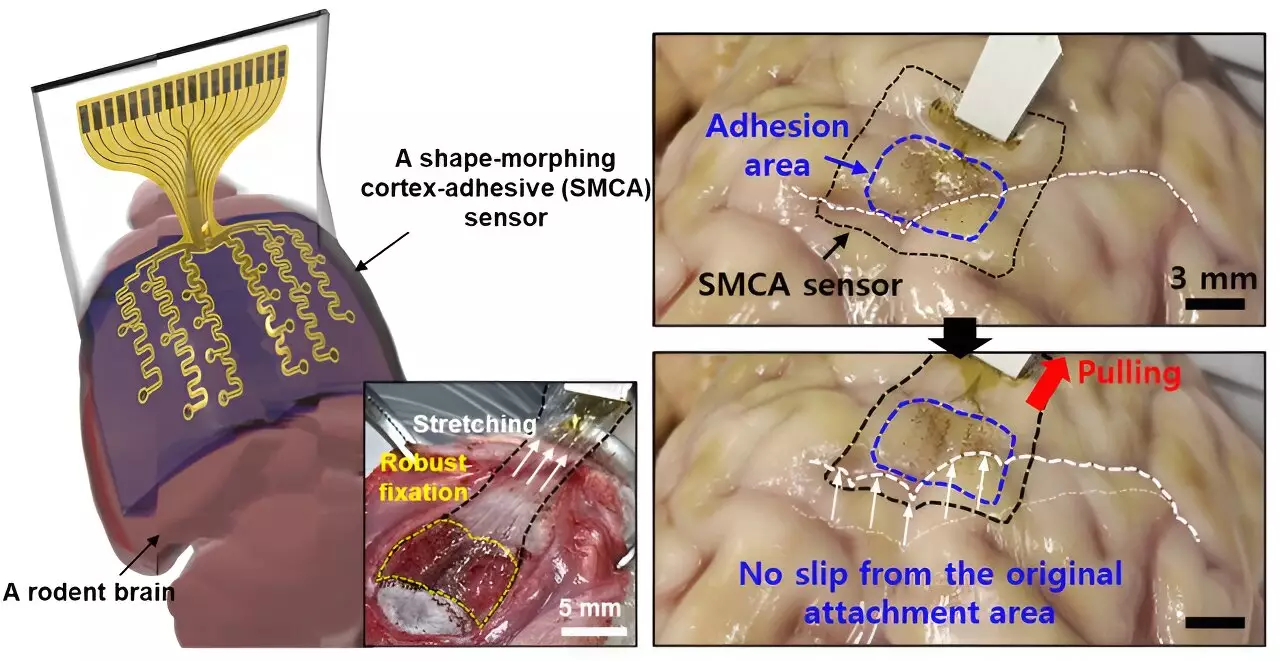
Donghee Son, the supervising author of the study, highlighted a prevalent issue in prior brain sensors: the challenge of conforming to the brain’s complex folds, which hindered accurate recording of brain signals. Traditional materials and designs often failed to achieve seamless adhesion, especially in areas of significant curvature. While earlier designs by Professors John A. Rogers and Dae-Hyeong Kim made strides in sensor thinness, they still struggled in maintaining adhesion on curvilinear surfaces, leading to potential inaccuracies in signal measurement.
The limitations observed in existing brain sensors—particularly their failure to adhere securely in highly curved areas—pose considerable obstacles in medical applications. These sensors often slipped from their intended positions, influenced by micro-movements in the brain and fluctuations in cerebral spinal fluid (CSF). Consequently, prolonged and precise monitoring and measurement efforts were severely hindered.
Yu’s team focused on overcoming these challenges, developing their innovative sensor, named ECoG, designed for strong and secure adhesion to brain tissue without creating voids that could introduce noise. This enhanced performance is particularly crucial for epilepsy treatment, where low-intensity focused ultrasound (LIFU) aims to reduce seizure activities.
One of the remarkable capacities of the new sensor lies in its ability to collect data in real-time. As many researchers strive to personalize treatment regimens for neurological conditions, the necessity of real-time brain wave monitoring becomes paramount. Son emphasized that earlier sensors fell short due to noise interference caused by ultrasound vibrations, making it immensely challenging to tailor treatment for individual patients accurately.
Breaking through this barrier, the dynamic form of the new sensor minimizes noise and allows for effective real-time monitoring, facilitating the creation of personalized ultrasound stimulation treatments. Such advancements mark a significant development toward individualized care, particularly vital in epilepsy management, where the variance in patient conditions complicates therapeutic practices.
The newly designed brain sensor comprises three innovative layers, each contributing significantly to its functionality. At the forefront is a hydrogel-based layer, promoting both physical and chemical bonding with brain tissue. Beneath this is a self-healing polymer layer capable of adapting its shape to conform tightly to the surrounding brain structures. The sensor concludes with a stretchable, ultrathin layer embedded with gold electrodes and interconnects.
Upon placement on brain tissue, the hydrogel layer initiates a gelation process, establishing a strong attachment instantly. Following this, the self-healing polymer layer begins to deform, enhancing contact with the brain’s contours and optimizing measurement capabilities. This mechanism highlights the importance of the sensor’s adaptability; as it transforms to fit the brain’s surface, it vastly improves signal fidelity.
The implications of this newly developed sensor are vast. Initial trials on awake rodents have demonstrated its efficacy in measuring brain waves and managing seizure activities. This encouraging evidence underscores its potential application beyond epilepsy to a broader spectrum of neurological disorders.
Moving forward, the researchers aspire to elevate this technology further by expanding the sensor’s capacity. Presently equipped with 16 electrode channels, there exists room for exhilaration through enhanced resolution and detailed brain signal mapping. Plans are in place to increase the density of electrodes, which may subsequently lead to superior diagnosis and therapy options for various neurological conditions.
The confluence of shape-morphing and high-adhesion technology in the innovative brain sensor indicates a transformative leap in neurology. With ongoing research and development, this endeavor holds the promise of reshaping efficacy in treatments for epilepsy and beyond, ultimately paving the way for personalized, effective medical solutions in neurology.