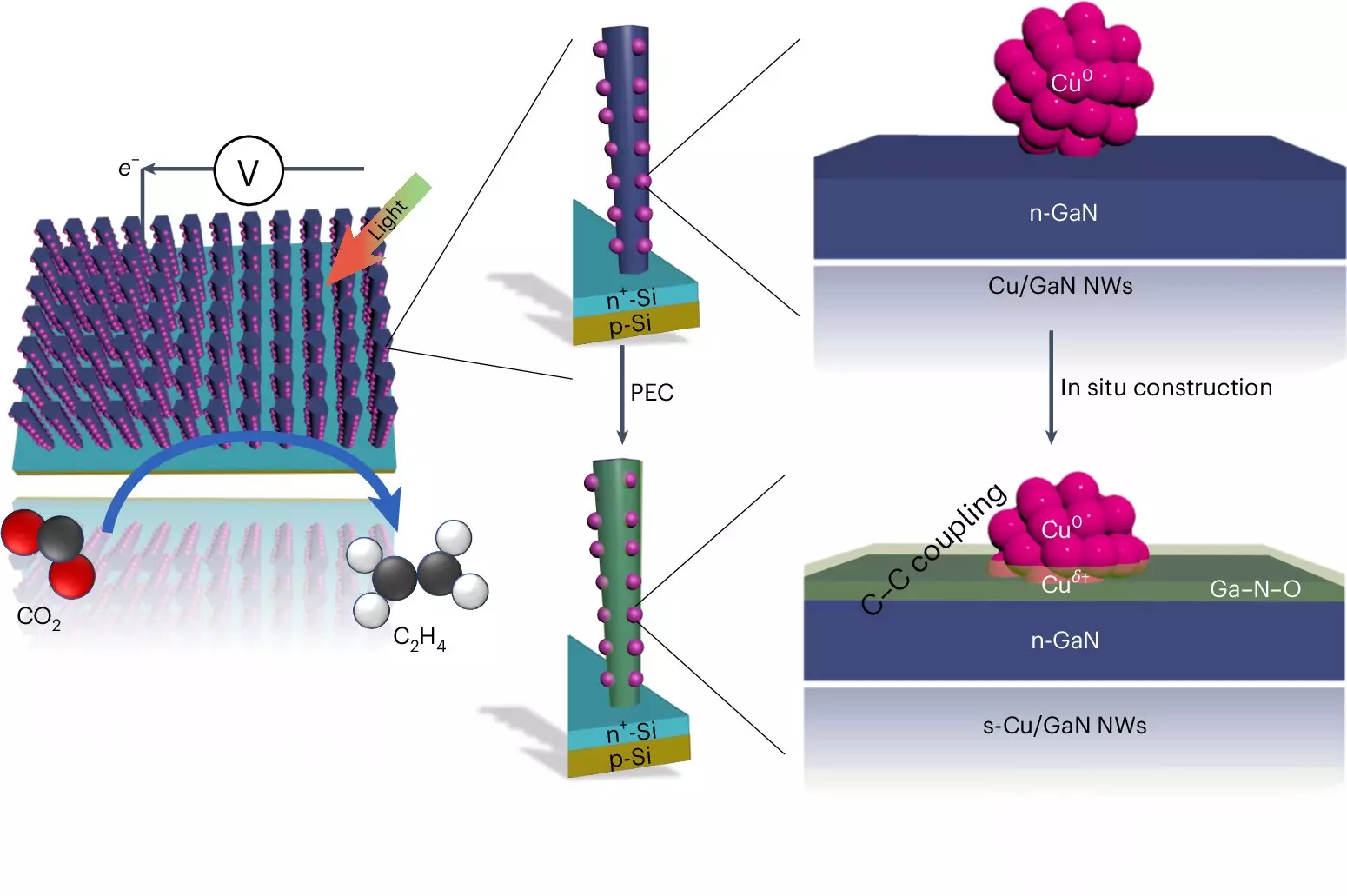
In the quest for sustainable energy solutions, one of the most promising avenues is the development of methods to recycle carbon dioxide (CO2) into valuable fuels. Researchers at the University of Michigan are at the forefront of this research, unveiling a groundbreaking artificial photosynthesis system that efficiently converts CO2 into ethylene, a hydrocarbon widely used in the production of plastics. This development not only enhances the utilization of CO2 but also addresses the pressing need for sustainable alternatives to fossil fuels in the chemical industry.
At the heart of this research is the innovative process of chaining carbon atoms, essential for synthesizing longer hydrocarbon molecules that could serve as liquid fuels. Ethylene constitutes the primary output of the system, and its production is achieved with efficiency that outstrips other conventional methods by a factor of five to six. The success of this artificial photosynthesis system lies in its ability to convert CO2, a greenhouse gas, into an economically valuable product while simultaneously mitigating environmental impact.
Zetian Mi, a leading professor in electrical and computer engineering at the University, highlights the significance of ethylene, stating that it is the world’s most produced organic compound. Typically derived from oil and gas through processes that involve extreme temperatures and pressures, the traditional manufacturing methods are unsustainable and high in emissions. The synthetic process developed by Mi’s team, therefore, presents a significant improvement over conventional methods.
The artificial photosynthesis device employs a dual semiconductor system combining gallium nitride nanowires and silicon, each playing a critical role in the conversion process. The gallium nitride nanowires, which are incredibly narrow, allow for an efficient absorption of sunlight, while the silicon base provides structural support for the reaction to take place.
Central to the production of ethylene is the reaction that occurs on copper clusters situated along the nanowires. Here, water and carbon dioxide interact under light exposure. The light energy splits water molecules, producing hydrogen and oxygen. The hydrogen is integral for forming the ethylene molecule, while the released oxygen interacts with the gallium nitride to enhance the process further. This synergy not only boosts efficiency but also establishes a self-healing property within the catalyst, enabling longer operation times without degradation.
A remarkable feature of the Michigan team’s device is its operational endurance. Previous systems, such as one built with silver and copper, displayed reasonable efficiency but suffered from rapid degradation, making them unsuitable for long-term use. In stark contrast, Mi’s artificial photosynthesis system has demonstrated an impressive runtime of 116 continuous hours, with similar devices operational for up to 3,000 hours. This durability is partially attributed to the advantageous interactions between the materials involved, allowing for a more resilient and effective catalytic environment.
The researchers reported that 61% of the electrons generated during the light-splitting process contributed directly to ethylene production, emphasizing the effectiveness of their system. By efficiently leveraging free electron flow, the device’s performance substantially surpasses that of competing technologies, which have demonstrated significantly lower production rates.
As the team looks toward the future, their ambitions extend beyond ethylene production alone. They aim to explore the synthesis of more complex hydrocarbons, such as propanol, which consists of three carbon atoms. Developing methods to produce a wider range of liquid fuels could enable existing transportation systems to transition toward sustainability, thereby addressing a key challenge in the fight against climate change.
The implications of this technology could be profound, offering a pathway to not only reduce greenhouse gas emissions but also create sustainable alternatives for key industrial products. The capacity to turn waste CO2 into useful fuels exemplifies the dual advantage of environmental benefit and economic opportunity, crucial in today’s rapidly evolving energy landscape.
The advances made by the University of Michigan team represent a significant leap toward making artificial photosynthesis a viable method for sustainable fuel production. As research continues, the potential to refine and expand these processes holds great promise for the future of energy and environmental quality.