Understanding biological systems through the lens of physics is an ongoing journey in science, as researchers continuously attempt to elucidate the complex interactions that govern life at the cellular level. In a groundbreaking study from São Paulo State University (UNESP), a novel approach links condensed matter physics concepts with protein compartmentalization, offering unique insights into cell dynamics. This work, spearheaded by Professor Mariano de Souza and Ph.D. candidate Lucas Squillante, proposes the existence of a Griffiths-like phase within cells, akin to the well-documented magnetic Griffiths phase observed in physical systems.
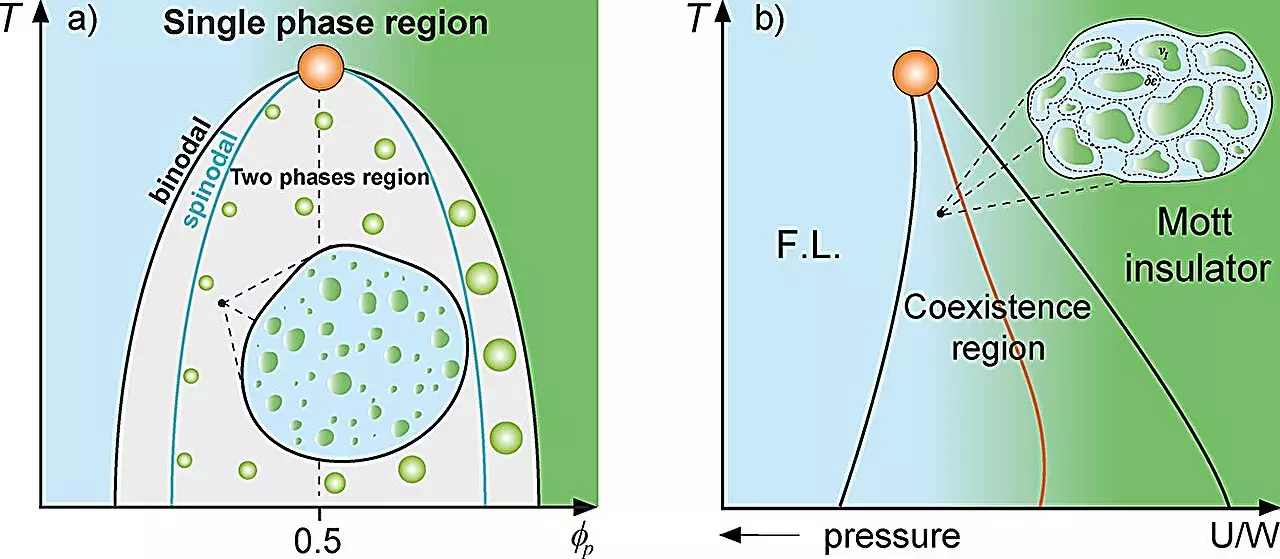
The foundational ideas from classical mixture theory provide a framework through which researchers can explore how distinct components within a biological system interact. The study at UNESP parallels well-known phenomena in condensed matter physics, wherein solid and liquid mixtures exhibit phase separation due to variances in density or magnetic orientation. The researchers draw parallels to scenarios like the Mott metal-insulator transition, wherein regions with contrasting properties coexist. This comparison underscores the potential for significant dynamics dampening due to “rare regions,” analogous to magnetized and non-magnetized areas that appear randomly in a medium.
Protein compartmentalization in cells, particularly the formation of protein droplets through liquid-liquid phase separation, mirrors these physical concepts. The study posits that under certain conditions, these droplets become “rare regions,” resulting in a slowed-down cellular environment that may significantly influence biological outcomes. The profound reduction in cellular dynamics observed near the binodal line, where phase separation occurs, could have far-reaching implications for how proteins interact and function within a cellular context.
The researchers utilized established thermodynamic models such as the Grüneisen parameter and the Flory-Huggins model to elucidate the protein compartmentalization dynamics. These analytical tools serve to quantify how different concentrations of proteins and solvent influence phase behavior within cells. The findings suggest a marked reduction in dynamic fluctuations as critical protein concentrations are approached, which not only impacts cellular function but may also hold keys to understanding how early life forms developed.
Notably, this alignment with the classical theories proposed by biochemist Aleksandr Oparin, who speculated on the origins of life through coacervates – simple droplet-like structures containing organic molecules – connects the dots between physical behavior and biological evolution. The capability of slower dynamics to facilitate the survival and evolution of primordial cellular structures is an exciting prospect, implying that dynamical properties critical for life have deep-rooted physical underpinnings.
The implications of these findings do not remain confined to theoretical realms. The researchers highlight potential links between liquid-liquid phase separation and several diseases. The study references how improper protein compartmentalization can lead to tumorigenesis, a process intimately tied to disease progression. Moreover, the formation of protein droplets has been implicated in neurodegenerative illnesses and even COVID-19, where the virus disrupts normal immune responses through phase separation mechanisms.
Professor Marcos Minicucci points out that understanding how these dynamic processes affect different diseases could lead to novel therapeutic interventions. Not only can insights from Griffiths-like phases provide diagnostic criteria, but they may also inform treatment strategies, illustrating a crucial intersection of physics and medicine. This interdisciplinary approach emphasizes the importance of collaboration across fields to tackle chronic health issues effectively.
The research emerging from UNESP paves the way for a richer understanding of biological systems through the principles of physics. By framing protein behavior within a Griffiths-like phase model, scientists can glean insights into both fundamental biology and its pathological states. The collaborative efforts of an international team bolster the notion that addressing complex questions necessitates diverse scientific expertise.
Future studies may involve deeper exploration into how variations in cellular environments affect these phases, especially as they relate to evolving understanding of protein roles in health and disease. As the dialogue between physics and biology expands, we are likely to witness innovative advancements in our grasp of life’s intricate workings, ultimately leading to practical applications that could revolutionize medicine and biotechnology. The path forward is certainly promising, laying the groundwork for transformative research that transcends disciplinary boundaries.